Approach: I sat down with Gaurav (the founder of ScienceIO) to understand his worldview. After several more hours of decoding research papers, I wrote this piece discussing cutting-edge healthcare technology ideas. Gaurav published it on LI and SubStack to demonstrate his expertise and get engagement from healthcare startups.
Note: This article was ghost-written for ScienceIO as part of my consulting services with Animalz to be published on their blog.
Large Language Models for Healthcare
Over 2 trillion gigabytes of healthcare data are generated annually — the majority of which are unstructured.
Structured health data can be stored in a database, analyzed in a spreadsheet or other tools, and shared with others. However, unstructured data, such as diagnostic reports, physicians’ notes, voice memos, and transcripts, aren’t readily usable.
Until recently, the best way to extract information from unstructured documents was to pay people to organize them. However, such manual methods fail to handle the vast volume of incoming healthcare data.
Old-school NLP systems help to a certain degree. For example, you could train a model to tag words (“acetaminophen” → “Drug” and “cancer” → “Disease”). But medical terms have lots of synonyms (“acetaminophen” == “Tylenol” == “paracetamol”), so you’d still need to map tags to ontologies so the data can be searched or analyzed.
As a result, NLP has had limited healthcare use cases and modest uptake. But that’s about to change. We’re on the brink of a new age in which LLMs will make healthcare records and documentation more usable and useful.
Here are 6 ways LLMs are set to transform healthcare:
1. LLMs are expert data labelers and coders
Models trained to label text using medical ontologies can transform documents into structured tables — faster than people can and with higher accuracy than old-school NLP or regex.
One of the first models we built was designed to find key terms in text and automatically map them to 20+ clinical ontologies in a few seconds. Given any medical document, it finds every medical condition, therapy, biomarker, and more. We aimed to demonstrate that healthcare documentation could be richly annotated using LLMs better and faster than before.
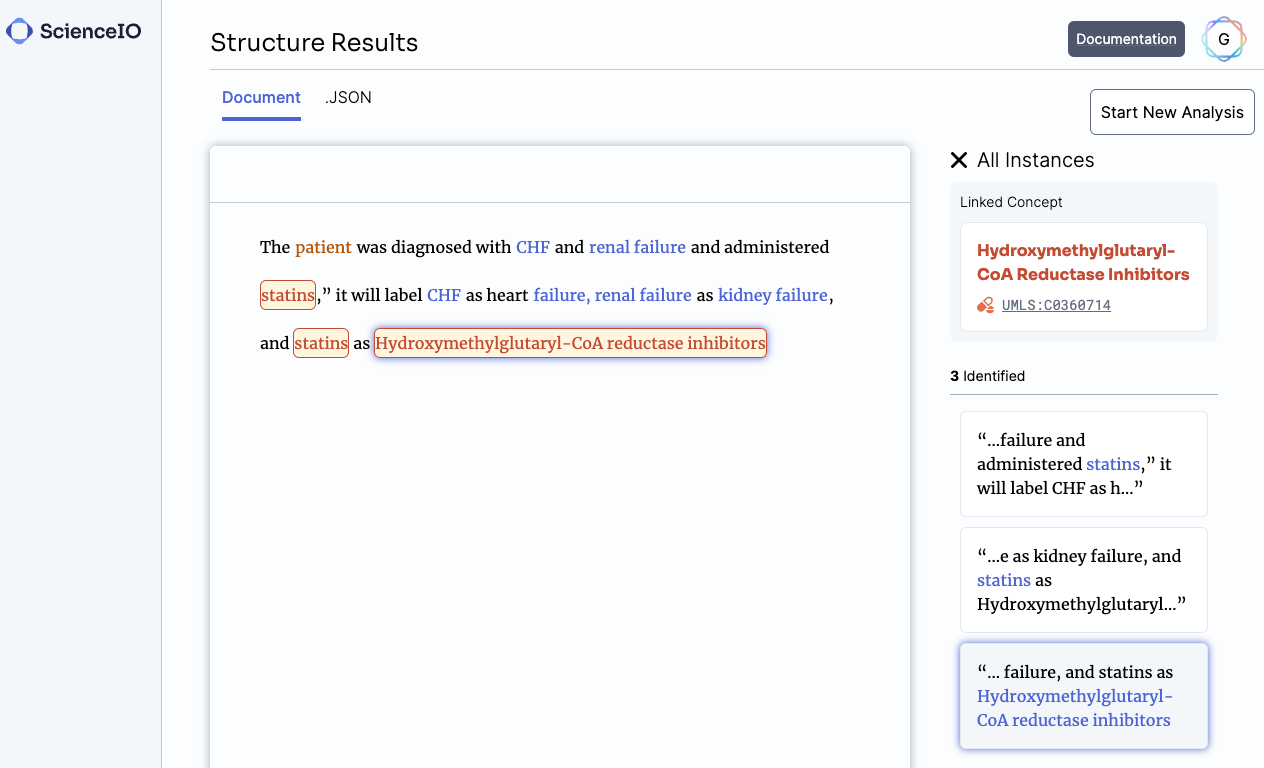
Data from unstructured text improves our understanding of patients
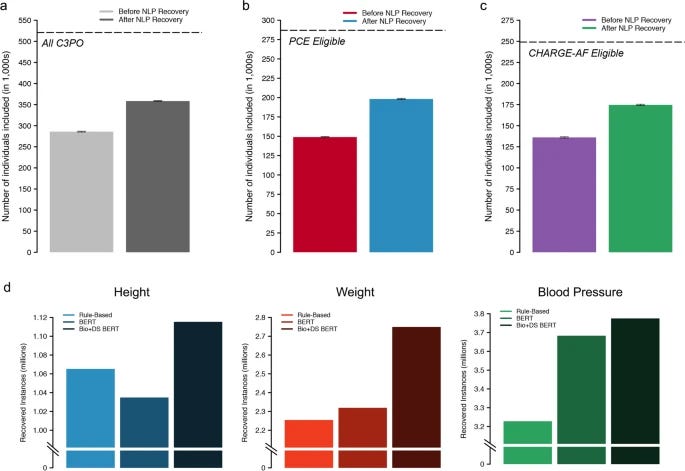
Last year, researchers from MGH and the Broad Institute published a study where they used a BERT-based model trained on discharge summaries to recover 31% of missing patient data (height, weight, systolic, and diastolic blood pressure). This study resulted in a less biased dataset for analyzing patient outcomes.
Data labeling LLMs can assist with medical coding
Here’s a demo of a ScienceIO LLM turns the transcript of a physician’s note into a list of medical codes:

Considerations:
Medical ontologies are often repetitive or redundant, especially in newer fields like precision medicine (UMLS has hundreds of entities related to a single gene). To build a reliable coding system, you need experts to curate what you want your model to train on and therefore produce.
Codes change frequently, particularly in billing, and thus the model must be re-trained or fine-tuned to keep pace.
If you want to use generative models for coding, know that they can “hallucinate” or create fake codes that look real. Encoder models are more accurate and reliable but are harder to adapt to new codes than generative models.
2. LLMs are patient privacy tools
HIPAA violations carry significant fines, and data breaches cost upwards of $10 million per incident. HIPAA anxiety has had many unintended consequences, the worst of which is that it prevents many teams from creating new solutions for patients out of fear of running afoul of HIPAA.
Trained properly, LLMs can find, redact, and flag protected healthcare information (PHI) or personally identifiable information (PII) much better than previous approaches (like regex).
We decided to develop our own healthcare-specific PHI LLMs after hearing from many customers that privacy tools built for other industries often remove vital clinical data when redacting data. In one case, general-purpose privacy tools removed every abbreviated medical condition and biomarker in a customer’s data, rendering the “private” data useless.
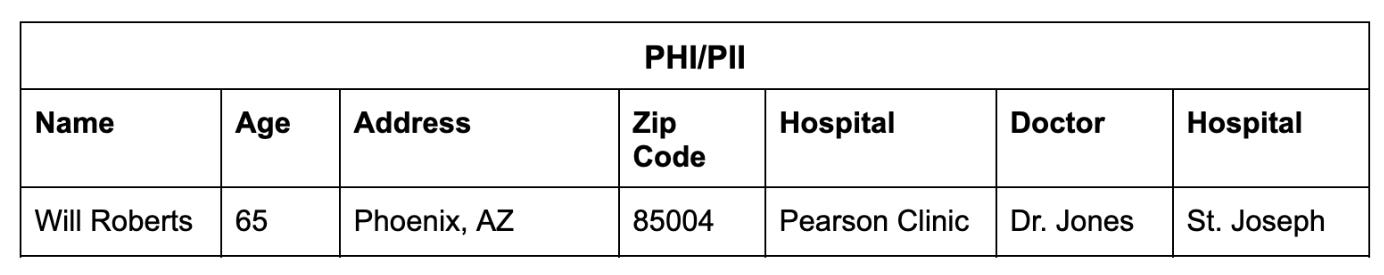
Consider this note:
Will Roberts is a 65-year-old male, residing in Phoenix, AZ, 85004, with a history of urinary tract infections. He presented to the Pearson clinic today with symptoms of dysuria and frequency. A urine culture was performed by Dr. Jones and showed significant growth of E. coli. The patient was started on a course of oral antibiotics and will follow up with the clinic in one week for a repeat urine culture. If no improvement, the patient will be referred to St. Joseph Hospital.
An LLM trained to find and classify PHI/PII can output a table like this:
Once the PHI is detectable, you can anonymize the record so it can be used responsibly while maintaining the integrity of the clinical information:
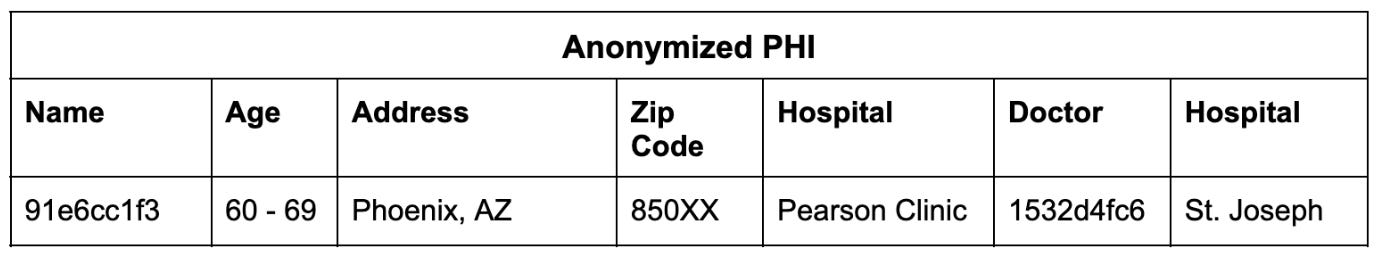
In this way, we can link multiple records to an individual and then anonymize the totality of their records.
Considerations:
– All algorithms have biases and LLMs are no exception. For example, they may fail to detect certain kinds of names (an early version of our PHI model misclassified every South Asian patient as a doctor, suggesting that the training data was curated by Asian parents). Therefore, bias testing is a critical part of our workflow.
We recommend not training PHI models with real patient information, especially gen AI models because doing so can ironically pose a privacy risk to the patients in the training data. The jury is still out on how to responsibly use patient records to train LLMs (we’ll share more in a future post).
3. LLMs are mathematical matchmakers
“85% of all clinical trials fail to recruit enough patients,” says Rosamund Round, Vice President of Patient Engagement at Parexel. “80% [of trials] are delayed due to recruitment problems and [high] dropout rates.”
Recruiting and retaining a single patient for a clinical trial costs hundreds of dollars. Trials recruiting patients with rarer attributes, such as a very specific biomarker, will pay much more—sometimes over $1M per patient. The challenge is even greater for trials with medications that have specific inclusion criteria—a new precision oncology drug will have a much smaller targetable population than a general anti-inflammatory drug, and thus, higher recruitment costs per patient.
LLMs can help trial sponsors and expand the pool of recruitable patients by matching trial criteria to patient attributes in the EMR.
There are a couple of ways to do this:
– LLMs trained using clinical information can represent both patient profiles and clinical trial criteria as vectors. Using these vectors, one can generate a list of candidate patients for a trial, or candidate trials for a patient very quickly.
– LLMs can find key terms in patient records to determine if they are a match (biomarkers, diagnostic criteria, vitals, test results). One benefit to this approach is that the “match” is more interpretable for a patient — you can search for patients based on specific observations, which a vector-based method cannot easily allow.
Last year, we used an LLM to analyze thousands of de-identified patient records on the Mayo Clinic Platform. Within 24 hours we found 55 patients who qualified for selective triple-negative breast cancer trials. None of these patients could be found by searching by ICD-10, CPT, or LOINC codes related to cancer.
Making trials visible to the patient or the care team can help solve for the top of the trial recruitment funnel, but only a small fraction of patients will enroll.
Generative LLMs can help with patient engagement to increase the probability of enrollment. LLMs can be used to explain a clinical trial’s goals, the candidate drug, how it works, what the trial entails, and other resources to engage patients and care teams. They can describe why the patient is a candidate and draft a note for the patient, explaining the personal opportunity and the impact they can make by enrolling.
A recent study in JAMA Internal Medicine had licensed healthcare professionals grade responses to patients’ questions, pitting ChatGPT against real physicians. They found that ChatGPT’s explanations were longer and more empathetic. This makes sense because ChatGPT is partially trained using peoples’ feedback on the quality of its outputs, so part of its goal is to appeal to users’ preferences. That these preferences on general tasks translated to a clinical context is only mildly surprising.
Still, I don’t recommend replacing doctors with chatbots. Instead, patient-centric solutions can have the best of both worlds—an LLM can generate possible responses, and care teams can select accurate and empathetic ones. Some cognitive burden is transferred to the model, and communication is still between the patient and their carer.
Considerations: A matching algorithm may have biases towards or against biases certain patient populations, indications, or even therapeutic modalities. These biases will impact the diversity and fairness of trial recruitment. We recommend testing any algorithm with data (real or synthetic) representing different patient populations to identify biases before real-world application, and ongoing.
4. LLMs are tireless automation machines
Administrative costs are estimated to be ~20% of national healthcare spend, or ~4% of the US GDP.
At a 2018 panel discussion, Donald Rucker, the former national coordinator for US Health IT, described a finding from a study he performed at Ohio State. “Of the 130,000 phone calls a day made by that 800-bed health institution, roughly half were 60 seconds or less, exchanging one fact.” Rucker envisioned EMRs as looking more like enterprise software where information exchange is less manual and more seamless.
LLMs are excellent at creating templated documentation. Reliable generative models can allow administrators to quickly draft information to be transferred, or even populate structured data fields from transcripts or voice memos.
As LLMs get increasingly plugged into other software and we get more tools that let them tap into data sources and APIs, they will become even more useful for automation. We’re rapidly heading towards an API-first health ecosystem, and that’s awesome for all the new data we’ll make, but what about the data that’s piled up? Translating between systems is tedious work, and no person wants to do it. LLMs acting as file-to-API and API-to-API translators can smooth out some of the rough edges.

Considerations:
Because healthcare information is sensitive and needs to be highly accurate, human supervision and reinforcement are necessary to achieve maximum quality control.
– I don’t see a future where massive GPT-X models are used universally across all use cases. Instead, smaller models trained specifically for complex tasks, which are easier to integrate into a private setting and easier to fine-tune, will prevail. For example, automated coding is possible with LLMs, but you want to use a model specifically trained on medical ontologies since, as we mentioned, general-purpose models often hallucinate codes.
5. LLMs are memory machines
On average, a physician in the US consults with over 2,000 patients annually.
Patients can have hundreds of pages of unstructured data and electronic health records, much of which can be repetitive. Many do not have the time and resources to absorb the full context of each patient’s history, let alone staying up to date on clinical trials and new research studies.
Fortunately, LLMs are highly effective data compression machines.
Transformers are designed to encode a lot of information compactly and efficiently, and they must learn to decode that encoding to produce coherent and contextually appropriate responses. Even smaller LLMs can learn from >1TB of text and produce weights in the GB regime.
This means LLMs can act as user-friendly knowledge bases. They provide information from the text they’ve been trained on and can do so in different styles or languages.

When provided with context, LLMs can also extract relevant information from it. This is particularly beneficial in the context of patient information retrieval. LLMs with the ability to pull data from records can assist in clinical workflows by summarizing a patient’s history, providing key data, and recommending potential tests or treatments based on its knowledge of the patient, treatment protocols, and research studies.
Here’s an example where LLMs provided a highlighted patient summary. A generative model produced the summary, and our encoder-based LLM detected all the key terms:

There are a lot of considerations to be made when relying on LLMs for information:
– As compression algorithms go, LLMs are lossy — they will not perfectly preserve all the original details of the text they were trained on and can produce inaccurate or imprecise answers. After all, many LLMs are only trained to produce coherent text, which is not necessarily accurate. Therefore, we do not recommend taking a model’s output at face value without confirming a reliable source.
Here’s a personal example: When I’ve used GPT-4 for a literature search of arXiv, >99% of the papers it gives me are real, but only ~40% of the source URLs are correct (~60% point to real but unrelated papers).
– Hallucination can be mitigated in many ways, including giving LLMs access to external databases, writing code to validate links, and/or rewarding them when they provide accurate sources. Monitor how search engines integrate LLMs and knowledge graphs for more accurate results.
6. LLMs are people simulators
LLMs can build a “representation” of individuals from its training data. Certain words are more likely to be associated with a person, and this statistical probability is encoded into the model.
This is why LLMs can provide relevant information on a person, like describing their biographical history or notable attributes — the words they generate are more likely to appear in the source text.
LLMs can also draw upon the shared attributes of individuals when generating text:

Thanks to this property, LLMs can serve as a statistical model informed by each patient’s experience. When trained on patient records, they will represent each patient, doctor, and other individual in the records.
Learning from each patient’s experience is challenging. Physicians take years to build an intuition for how to diagnose and treat patients. This intuition is refined with each patient encounter and as medicine advances. LLMs approximate that process in a fraction of the time and may develop emergent capabilities that might be useful for patient care.
What would you do if you could draw on the history of a million patients in realtime?
For a long time, I’ve been fascinated by the idea of a patient atlas — an algorithm that, when given a limited amount of info about a patient, could produce a differential diagnosis, assign probabilities to each diagnosis, suggest interventions, tests, or treatments, and refine its working model of them as it learns more.
Given the rapid pace of artificial intelligence, I don’t believe these capabilities are far out.
Considerations:
LLMs trained on real patient data will remember real patient details, which poses a serious privacy risk to those patients. This problem is much worse when you train a neural network on tabular data (even a modest GAN can fully memorize most clinical datasets in as little as 2 epochs). Anonymized datasets covering a wide swath of the patient population will be crucial.
It’s important to remember that LLMs learn only from documented signals, whereas physicians often pick up on nuanced signals that no EHR captures.
In the near term, I believe LLMs will be most useful in reducing the cognitive burden for care teams, patients, operators, and developers. LLMs save time by performing long and tedious that require no special expertise, and they can save energy by supporting quick tasks that require mental effort.
In the longer term, I genuinely believe that we can reshape the way healthcare works, and AI-driven products purpose-built for healthcare will play a big part. In our post on the last 15 years of healthcare, we reflected that healthcare is capable of incredible transformation given the right conditions. LLMs are a powerful tool we can use to improve healthcare if we think about how to do the right jobs, not just do the same ones faster or cheaper.
That’s the change we’re building for.
Note: This article was ghost-written for ScienceIO as part of my consulting services with Animalz to be published on their blog.